
When someone with a cataract makes the decision to undergo modern cataract surgery, there is yet one more decision that must be made: what type of intraocular lens (IOL) to have placed in the eye. Most eye surgeons (or their surgical schedulers) will discuss the various options available. These may include monofocal, toric, multifocal, pseudo-accommodating, and extended depth of focus (EDOF) IOLs. Such a broad range of choices can be a bit overwhelming. It was not always so.
As recently as the turn of the millennia patients were not given a choice as to what type of IOL to have implanted as there was no choice to be made. Only one type of IOL, “monofocal” was used. A half century prior even that option was not available.
That all changed with the pioneering work of Sir Harold Ridley, who first implanted an IOL in 1949. Although he is now considered a hero in the field of ophthalmology, he was initially demonized by many of his contemporary surgeons for placing a foreign object into the eye. Sir Harold Ridley’s early critics, however, had reason to be concerned. At that time it was simply not known what would happen to a foreign material implanted in the eye.
Today an intraocular lens (IOL) is almost always placed in the eye at the time of surgery. They are generally considered to be both safe and effective as all IOLs implanted in the USA undergo a rigorous process of evaluation by the FDA. Nonetheless, modern eye surgeons know that not all material choices have proven to be long-lasting. It is possible for the IOL material to degrade after it has been implanted in the eye.
Photo: Cataract Surgery (Part 4 of 4) | Dr. David Richardson via YouTube
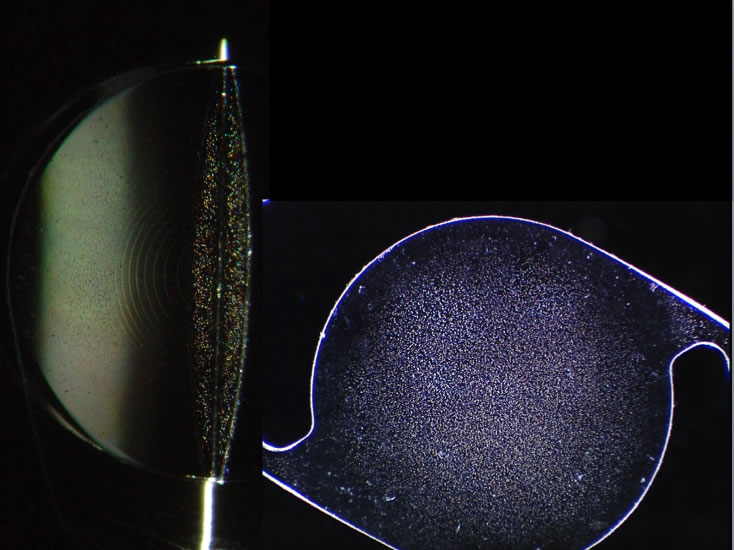
Photo: Glistenings on IOL | Photos 1 & 3 Credit: www.iolsafety.com | Photo 2 Credit: crstoday.com
One of the most commonly chosen IOLs by surgeons in both Canada and the USA is the Alcon AcrySof® IOL. This IOL is made of a soft acrylic material that allows it to be injected into the eye through a small (less than 3mm) incision. Recently, however, reports of significant “sub-surface nano-glistenings” have been observed in AcrySof® IOLs years after they have been implanted in the eye.
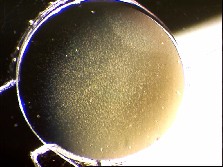
Glistenings (or nano-glistenings) are microscopic fluid-filled bubbles that can form in synthetic lens material. A small number of glistenings may be observed in any IOL that has been in the eye over a period of time. If these glistenings were only visible to the eye surgeon under the microscope and caused no problems with vision then they would be an interesting footnote in some journal of material science. However, there is now evidence that large amounts of these glistenings may indeed degrade the quality of vision1. Night vision, in particular, is reduced by glistenings as they can result in glare around lights.
The material used in the AcrySof® IOL has a tendency to develop more of these glistenings than is seen in IOLs made of other materials. Indeed, by only a few years after cataract surgery the majority of AcrySof® IOLs will likely have developed these glistenings2. Over time it appears that these glistenings only get worse3.
In a review of the Alcon AcrySof® lens by the US Department of Health and Human Services4:
- We conclude that the AcrySof® Natural IOLs do not demonstrate substantial clinical benefit in comparison with currently available IOLs [emphasis mine] (p. 1010)
- Moreover, in our review…regarding the blue light filtering optic, we found evidence suggesting that the blue-filtering lenses could decrease best possible vision. (p. 1006)
- the glistenings associated with AcrySof® Natural lenses that develops overtime causes disability glare rather than reduces it. [emphasis mine] (p. 1009)
The International Society for Intraocular Lens Safety has published a very succinct review of the literature concerning these glistenings. Here is the conclusion of this document:
“The evidence is overwhelming that glistenings in AcrySof® intraocular lenses have an adverse influence on vision [emphasis is mine]…the use of these lenses in cataract surgery patients should be reconsidered.”
Photo: Simulation of effect of glistenings on night time vision. | Photo Credit: www.iolsafety.com
To my way of thinking, the above statements are so compelling as to warrant serious reflection about whether Alcon AcrySof® IOLs should be implanted at all (or at least in younger patients) if there is a same-diopter IOL option available in another material. When choosing an IOL (as with every treatment recommended by a physician), “primum non nocere” (above all, do no harm) should be a guiding principal. Given what we now know about the Alcon AcrySof® IOL, it is my opinion that choosing the Alcon AcrySof® IOL (when a same-diopter IOL is available in another material) violates this fundamental principal of practicing medicine.
When choosing an IOL (as with every treatment recommended by a physician), “primum non nocere” (above all, do no harm) should be a guiding principal.
To be fair, not all cataract surgeons are aware of the issue of glistenings. There is a never-ending stream of announcements, articles, and studies related to eye disease that no human could reasonably be expected to keep up with. Even those with knowledge of glistenings may be under the impression that they do not have a noticeable impact on the quality of vision.
Patients, however, entrust their eye surgeons to make the best decision in the hopes that they will enjoy many years of excellent vision. We now know that the AcrySof® material may result in decreased vision over time. As such, the case against implanting the AcrySof® IOL is especially strong in younger patients who are more likely to suffer from glistenings during their lifetime. Furthermore, given that “AcrySof® Natural IOLs do not demonstrate substantial clinical benefit in comparison with currently available IOLs” there seems to be no compelling reason to implant the AcrySof® IOL in patients of any age.
Ultimately, it is the patient who must live with whatever material is implanted. Unlike with “premium” IOLs (e.g., multifocal, toric, etc.) patients are not routinely informed about the brand and model of the IOL when they elect to have an insurance-covered monofocal IOL implanted. This choice is generally made by the surgeon (or occasionally even the surgery center or HMO) without any discussion beyond “at the time of cataract surgery your cataract will be removed and replaced with a plastic lens”.
There is a lot of information that must be conveyed prior to cataract surgery. Often the surgeon will demonstrate how surgery is done, warn of the risks, and discuss the type of IOL to be used. By that time many patient’s eyes are glazing over with information overload.
It’s understandable why this would be the case. There is a lot of information that must be conveyed prior to cataract surgery. Often the surgeon will demonstrate how surgery is done, warn of the risks, and discuss the type of IOL to be used. By that time many patient’s eyes are glazing over with information overload. Reviewing the nuances of IOL material choice just doesn’t seem necessary or prudent at that point. After all, most IOL materials don’t have definite clinical benefits or downsides. However, for surgeons whose “go to” IOL is the AcrySof® IOL a simple handout could be offered to the patient to be read at his or her leisure.
Summary
If there is a known issue with the material used in a surgical implant I am confident many (if not all) patients would want to be made aware of it prior to it being placed in the body. In the case of the AcrySof® IOL there is a known issue (high likelihood of glistenings) that may degrade at least night vision over time. Alternatives to using the AcrySof® IOL are readily available and provide similar clinical benefit to the patient without the high risk of developing glistenings.
Surgeons have their preferences and there may be reasons why an individual surgeon feels that the choice of an AcrySof® IOL is still the best choice for a given patient despite the risk of glistenings. When there are potential advantages to a given implant despite known issues with the material then the patient and surgeon may reasonably elect to move forward with that implant. It should, however, be an informed decision.
References:
- Matsushima H, Nagata M, Katsuki Y, et al. Decreased visual acuity resulting from glistening and sub-surface nano-glistening formation in intraocular lenses: A retrospective analysis of 5 cases. Saudi Journal of Ophthalmology. 2015;29(4):259-263. doi:10.1016/j.sjopt.2015.07.001.
- Christiansen G, Durcan FJ, Olson RJ, Christiansen K. Glistenings in the AcrySof® intraocular lens: pilot study. J Cataract Refract Surg. 2001;27(5):728-33.
- Behndig A, Monestam E. Quantification of glistenings in intraocular lenses using Scheimpflug photography. J Cat Refract Surg 2009;35:7-14.
- DEPARTMENT OF HEALTH AND HUMAN SERVICES, Centers for Medicare & Medicaid Services, 42 CFR Parts 410, 411, 412, 413, 416, 419, and 489 [CMS-1504-FC and CMS-1498-IFC2], RIN 0938-AP82 and RIN 0938-AP80, published Nov 2010; available at http://www.ofr.gov/OFRUpload/OFRData/2010-27926_PI.pdf
About the Author:
 Dr. David Richardson has performed thousands of cataract surgeries without the need for laser assistance. Although he finds Femto technology to be interesting he is far from convinced that there is any real benefit to his patients. As such, he has chosen not to recommend this technology to his patients who need cataract surgery.
Dr. David Richardson has performed thousands of cataract surgeries without the need for laser assistance. Although he finds Femto technology to be interesting he is far from convinced that there is any real benefit to his patients. As such, he has chosen not to recommend this technology to his patients who need cataract surgery.











Recent Comments